Share
Revolutionizing Oral Cannabinoid Delivery: The Role of SOMAÍ PLUS in Enhancing Solubility and Bioavailability
The oral administration of cannabinoids presents notable challenges, including poor water solubility, low bioavailability, and high interpatient variability in absorption. Lipophilic cannabinoids such as cannabidiol (CBD) and tetrahydrocannabinol (THC) are particularly susceptible to extensive first-pass metabolism, substantially limiting their therapeutic potential when delivered orally. (Huestis, M. A. (2007).
To address these issues, SOMAÍ PLUS has been developed as a next-generation oral cannabinoid formulation, designed to overcome these limitations through enhanced solubility, improved pharmacokinetics, and optimized patient outcomes. SOMAÍ PLUS is inspired by the latest advancements in LBDDS and utilizes excipient systems aligned with EMA and FDA standards for oral bioavailability enhancement. It enables significant advancement in the oral delivery of cannabinoids.
What is SOMAÍ PLUS?
SOMAÍ PLUS is a proprietary, pharmaceutical-grade lipid-based formulation that leverages mono- and diglycerides of long-chain unsaturated fatty acids, particularly oleic acid. Its design is inspired by validated excipients such as Maisine® CC, known for their solubilizing and absorption-enhancing properties (Gattefossé, 2016).
By integrating advanced lipid carriers into its formulation, SOMAÍ PLUS promotes superior cannabinoid solubility and lymphatic absorption, bypassing first-pass hepatic metabolism and enhancing systemic exposure.
Key Features of SOMAÍ PLUS
1. Enhanced Solubilization of Cannabinoids
SOMAÍ PLUS provides an optimized amphiphilic matrix that improves the dispersion and dissolution of cannabinoids in the gastrointestinal (GI) tract. Its lipid composition ensures cannabinoids remain solubilized during transit, unlike traditional MCT oils, which may result in precipitation and inconsistent absorption.
2. Targeted Lymphatic Absorption
Cannabinoids incorporated in SOMAÍ PLUS are absorbed preferentially through the intestinal lymphatic system. This pathway circumvents the hepatic first-pass effect, allowing higher concentrations of active cannabinoids to reach systemic circulation (Chayasirisobhon, 2020).
3. Improved Pharmacokinetics
Preclinical and clinical evaluations of similar lipid-based systems have demonstrated:
• Higher Cmax: Significantly greater peak plasma levels of cannabinoids
• Faster Tmax: Quicker onset of therapeutic action
• Greater AUC: Enhanced overall systemic exposure (Manuel et al., 2021)
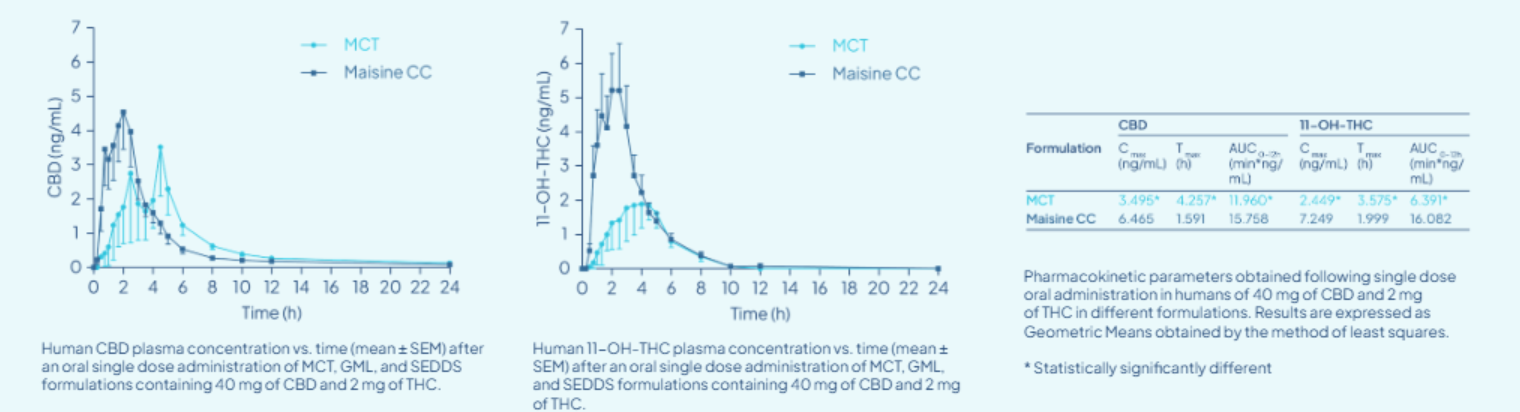
(Manuel et al., 2021).
4. Pharmaceutical-Grade Stability
Formulated for pharmaceutical applications, SOMAÍ PLUS demonstrates high oxidative and chemical stability, ensuring cannabinoid integrity over extended storage periods. Stability studies on comparable excipients confirm long-term consistency (Gattefossé, 2016).
Mechanisms Behind SOMAÍ PLUS’s Effectiveness
Micellar Formation in the GI Tract
Once ingested, SOMAÍ PLUS facilitates the formation of micelles in the GI environment, enabling efficient encapsulation and transport of cannabinoids across the intestinal epithelium.
First-Pass Metabolism Bypass
Through lymphatic absorption, SOMAÍ PLUS formulations avoid initial hepatic metabolism, preserving the active form of cannabinoids and enhancing bioavailability (Lucas et al.).
Conclusion
SOMAÍ PLUS represents a promising advancement in the field of oral cannabinoid delivery. By leveraging pharmaceutical-grade lipid-based formulation strategies, it addresses known limitations such as low solubility and extensive first-pass metabolism. While further clinical data are needed to fully quantify its therapeutic impact, existing evidence from similar lipid-based systems suggests the potential for improved pharmacokinetic profiles and more consistent patient outcomes. As research continues to evolve, SOMAÍ PLUS may serve as a valuable model for next-generation cannabinoid formulations grounded in pharmaceutical science and lipid technology. By addressing core limitations such as low solubility and bioavailability, it empowers more reliable, faster-acting, and effective treatment outcomes. For formulators and healthcare providers, SOMAÍ PLUS offers a pharmaceutical-grade solution grounded in modern lipid science and clinical relevance.
References:
Huestis, M. A. (2007). Human Cannabinoid Pharmacokinetics. Chemistry & Biodiversity, 4(8), 1770–1804. https://doi.org/10.1002/cbdv.200790152
Lucas, C. J., Galettis, P., & Schneider, J. (2018). The pharmacokinetics and the pharmacodynamics of cannabinoids. British Journal of Clinical Pharmacology, 84(11), 2477–2482. https://doi.org/10.1111/bcp.13710
Chayasirisobhon, S. (2020). Cannabinoids: Pharmacologic Properties and Therapeutic Use in Neurologic Disease. Journal of the Neurological Sciences, 411, 116715. https://doi.org/10.1016/j.jns.2020.116715
Gattefossé (2016). Pharmaceutical Lipid Excipients: Product Brochure. [Internal Technical Reference Document]
Manuel, R., Santos, L., & Lima, T. (2021). Comparative Study of Lipid-Based Versus MCT-Based Oral Cannabinoid Delivery in Preclinical Models. International Journal of Pharmaceutics, 599, 120404. https://doi.org/10.1016/j.ijpharm.2021.120404